Platforms

Technologies
As platform-independent bioanalytical CRO, we support precision medicine through translational sciences. Our integrated Platforms – AutoTIL™ Microtumors, Hyperplex™ Proteomics and QSM™ Metabolic Phenotyping – provide high-resolution, predictive data that translate preclinical findings into actionable insights for clinical development.
AutoTIL™ Microtumors - Real Tumors. Real Immunity. Real Responses.
Patient-Derived Tumor Models with Immune Competence
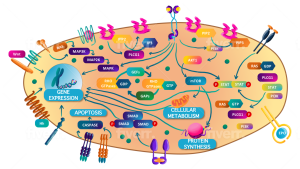
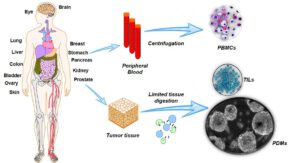
AutoTIL™ Microtumors preserve the patient’s native tumor architecture, cellular heterogeneity, and autologous immune cells, providing physiologically relevant models for preclinical cancer research.
Immune-Oncology Insights
Integrating tumor-infiltrating lymphocytes (TILs) and tumor-associated macrophages (TAMs), AutoTIL™ enables evaluation of checkpoint inhibitors, immunomodulators, adoptive cell therapies, and combination strategies in a native tumor context. This helps generate clinically meaningful insights and improves translational predictivity.
Ready-to-Use and Broadly Applicable
Non-cultured and ready within one week of biopsy, AutoTIL™ Microtumors accelerate preclinical testing without engraftment or modification. They are compatible with all solid tumor types, supporting mechanism-of-action studies, biomarker validation, and drug response assessment across diverse cancers.
Multi-Omics and Mechanistic Understanding
These models support transcriptomics, proteomics, metabolomics, and other multi-omics analyses, providing detailed mechanistic insights into tumor biology, immune interactions, and resistance mechanisms.
Translational Impact
By combining patient specificity, immune competence, and preserved tumor architecture, AutoTIL™ Microtumors improve prediction of clinical responses, guide combination therapy strategies, and support biomarker discovery, accelerating immune-oncology-focused drug development.
Hyperplex™ – Advanced Multiplex Protein Profiling for Cancer Research
Unlock deeper biological insights with our next-generation protein profiling platform – designed to accelerate discovery and development in oncology and beyond.
Comprehensive Protein Insights from Limited Material
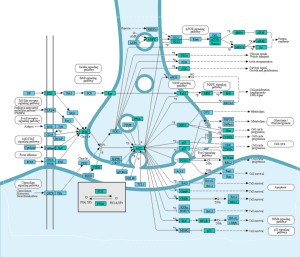
We profile up to 1,500 proteins—phospho and total—in a single assay using only 20–60 μg of sample. This enables deep, high-throughput data generation even from scarce or valuable clinical material, helping you validate targets and mechanisms more efficiently.
Reproducible, Decision-Ready Data
Our service combines automated electrophoresis with bead-based assays to deliver highly accurate, reproducible quantification. With 1,500+ validated antibodies and support for custom panels, you receive robust data you can rely on in therapeutic development.
Applicable Across Models and Stages
From 2D/3D cultures and organoids to xenografts, PDX, and tissue samples, our services integrate seamlessly into both preclinical and clinical research workflows, supporting diverse study designs in oncology.
Actionable Results for Oncology Programs
We deliver results as heatmaps, volcano plots, and pathway maps—making it straightforward to identify signaling changes, resistance mechanisms, and potential therapeutic strategies.
Supporting Faster, Smarter Development
Our end-to-end service reduces experimental risk, accelerates data-driven decision-making, and helps move oncology programs more efficiently toward the clinic.
QSM™ – Quantitative System Metabolism for Precision Bioenergetics
Comprehensive Metabolic Profiling
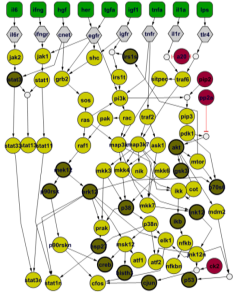
QSM™ quantifies metabolite levels and pathway activity across 22 key pathways, including ATP production, mitochondrial respiration, glycolysis, and OXPHOS. It works with clinically relevant material, including scarce samples and FFPE tissues, providing deep insights into tumor-specific metabolic dependencies and pharmacodynamic markers to support translational decision-making.
Functional Insight Through Modeling
By integrating LC-MS–based proteomics with kinetic metabolic models, QSM™ captures enzyme activity and regulatory dynamics, revealing pathway regulation and potential vulnerabilities. This allows researchers to optimize preclinical experiments, stratify patient populations, and generate robust functional data to guide therapy development.
Broad Sample Compatibility
Validated for single cells, cell cultures, tissues, and FFPE specimens, QSM™ adapts seamlessly across experimental systems without compromising data quality. This flexibility ensures that findings can be translated from model systems to clinical contexts, even when sample availability is limited.
Predictive and Translational Value
Metabolite and pathway data can be integrated with PK/PD and kinetic models, improving the interpretation of drug effects. Researchers gain validated biomarkers, clearer therapy response insights, and stronger foundations for model-informed drug development, enhancing the translational impact of preclinical studies.
Supporting Translational Oncology
QSM™ delivers detailed metabolic profiling that can inform fluxomics studies, pharmacometric analyses, and quantitative systems pharmacology models. By providing high-quality, centralized metabolic data, it aids in identifying resistance mechanisms, predicting therapy responses, and enhancing the value of oncology R&D, thereby accelerating the transition from discovery to clinical application.
Want to Talk to a scientist?
We help you identify your pathways and marker of interest.